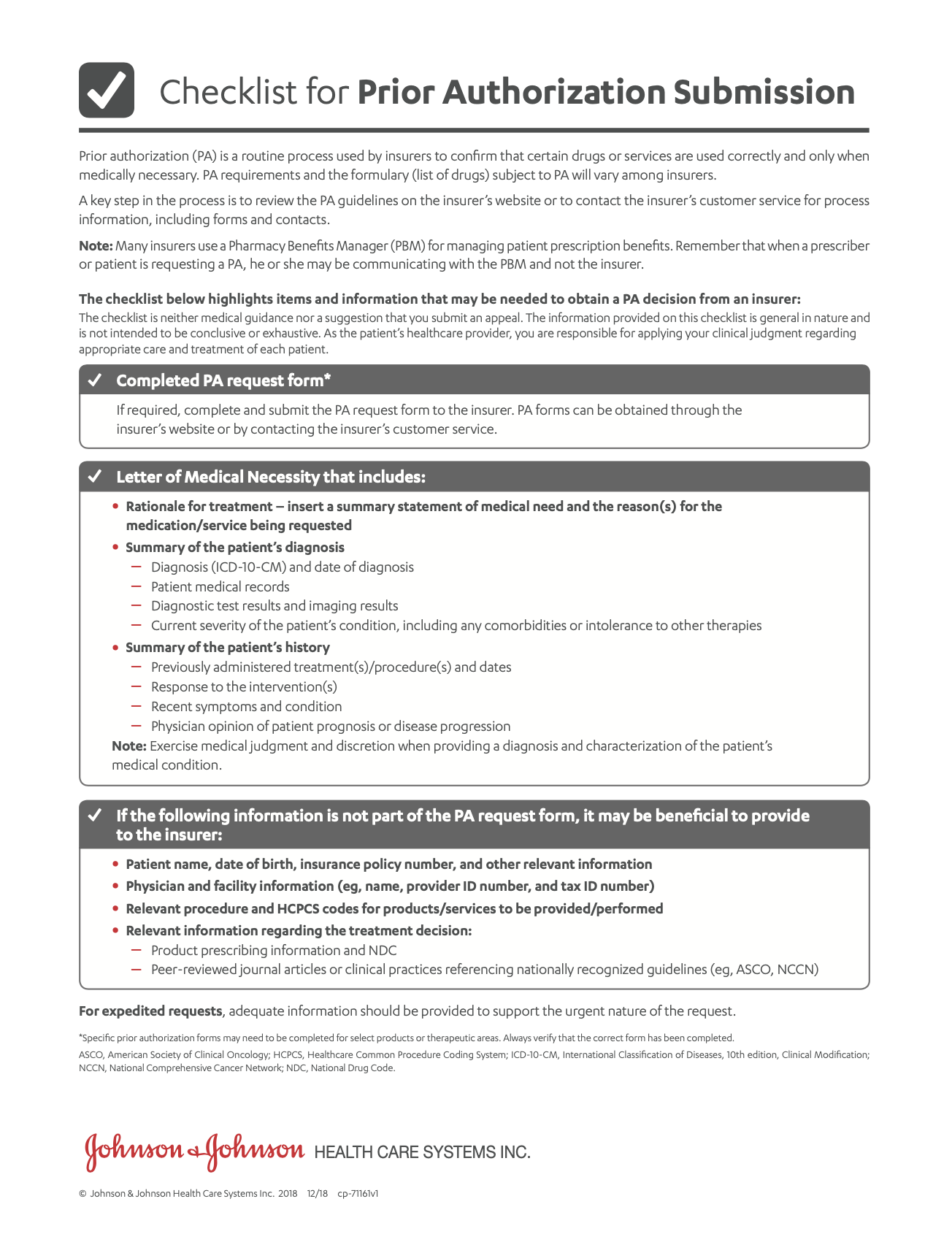
Helping Patients Afford EDURANT®
- Janssen CarePath Resource GuideA comprehensive summary of support tools for your office to help patients start and stay on treatment.
- Letter of Medical NecessityA template that you can fill out and submit to a patient’s health insurance provider. You may use it to explain why EDURANT® is medically necessary for your patient.
- Patient Affordability OptionsDiscover options that can make EDURANT® more affordable for your patients.
- Savings Program OverviewEligible patients using commercial or private insurance can save on out-of-pocket costs for EDURANT®.
- Savings Program Rebate FormA form the patient can submit if the pharmacy isn’t able to process the Janssen CarePath Savings Program card.

Helping Patients Afford EDURANT®
Select your patient’s coverage status for relevant resources.
For Patients with Commercial or Private Insurance
There is a limit to savings each year. Savings may apply to co-pay, co-insurance, or deductible.
Patients may participate without sharing their income information.
We provide cost support directly to patients through the Janssen CarePath Savings Program. This benefit is intended to help eligible patients afford their out-of-pocket obligations as set by their health plans. The cost support is meant solely for patients—not health plans and/or their partners.
If your patients are having any difficulty accessing cost support through the Janssen CarePath Savings Program, please have them contact us at 877-CarePath (877-227-3728).
Express Enrollment at JanssenCarePathPortal.com/express
Providers can check patients' eligibillity and enroll eligible patients in the Janssen CarePath Savings Program with no Business Associate Agreement (BAA) required.
Mobile Enrollment for the Janssen CarePath Savings Program for Patients
Your patients can use their mobile phone to enroll in the Savings Program. They can text “SAVINGS” to 89633 (message and data rates may apply*) or visit MyJanssenCarePath.com/Express to receive an electronic card that can be saved to their mobile wallet.
*Terms and Privacy Policy can be found at JanssenCarePath.com/Terms-Conditions-Mobile and JanssenCarePath.com/Privacy-Policy.
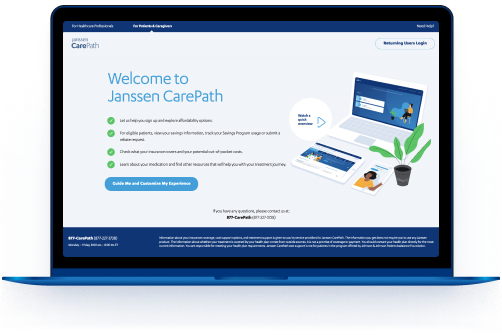
Janssen CarePath for Your Patients
Your patients can visit MyJanssenCarePath.com to sign up for the Savings Program. Once they’ve created an account, they can sign up for personalized treatment support. They can also get alerts, updates, and even manage their benefits 24/7.
Talk to your patients about
MyJanssenCarePath.com
In addition to the Janssen CarePath Savings Program, here are some independent programs that may be right for your patients.
Additional Affordability Support from Janssen
For Patients with Government Coverage
Even if your patients have government coverage like Medicare, we can identify programs that may help them afford their medications. Here are some independent programs that may be right for them.
- Pay their monthly premiums
- Reduce or eliminate their deductible
- Reduce or eliminate their co-insurance and co-payments
- Have no gap in coverage
Additional Affordability Support from Janssen
For Patients with No Insurance Coverage
If your patients need help with drug costs, we can identify programs that may help them afford their medications.
Here are some programs that are not offered by Janssen. Each program has its own eligibility rules.
Take a look and see which ones may be right for your patients.
Uninsured Patients May Be Eligible for Additional Support
There is a limit to savings each year. Savings may apply to co-pay, co-insurance, or deductible.
Patients may participate without sharing their income information.
We provide cost support directly to patients through the Janssen CarePath Savings Program. This benefit is intended to help eligible patients afford their out-of-pocket obligations as set by their health plans. The cost support is meant solely for patients—not health plans and/or their partners.
If your patients are having any difficulty accessing cost support through the Janssen CarePath Savings Program, please have them contact us at 877-CarePath (877-227-3728).
Express Enrollment at JanssenCarePathPortal.com/express
Providers can check patients' eligibillity and enroll eligible patients in the Janssen CarePath Savings Program with no Business Associate Agreement (BAA) required.
Mobile Enrollment for the Janssen CarePath Savings Program for Patients
Your patients can use their mobile phone to enroll in the Savings Program. They can text “SAVINGS” to 89633 (message and data rates may apply*) or visit MyJanssenCarePath.com/Express to receive an electronic card that can be saved to their mobile wallet.
*Terms and Privacy Policy can be found at JanssenCarePath.com/Terms-Conditions-Mobile and JanssenCarePath.com/Privacy-Policy.

Janssen CarePath for Your Patients
Your patients can visit MyJanssenCarePath.com to sign up for the Savings Program. Once they’ve created an account, they can sign up for personalized treatment support. They can also get alerts, updates, and even manage their benefits 24/7.
Talk to your patients about
MyJanssenCarePath.com
In addition to the Janssen CarePath Savings Program, here are some independent programs that may be right for your patients.
- Pay their monthly premiums
- Reduce or eliminate their deductible
- Reduce or eliminate their co-insurance and co-payments
- Have no gap in coverage