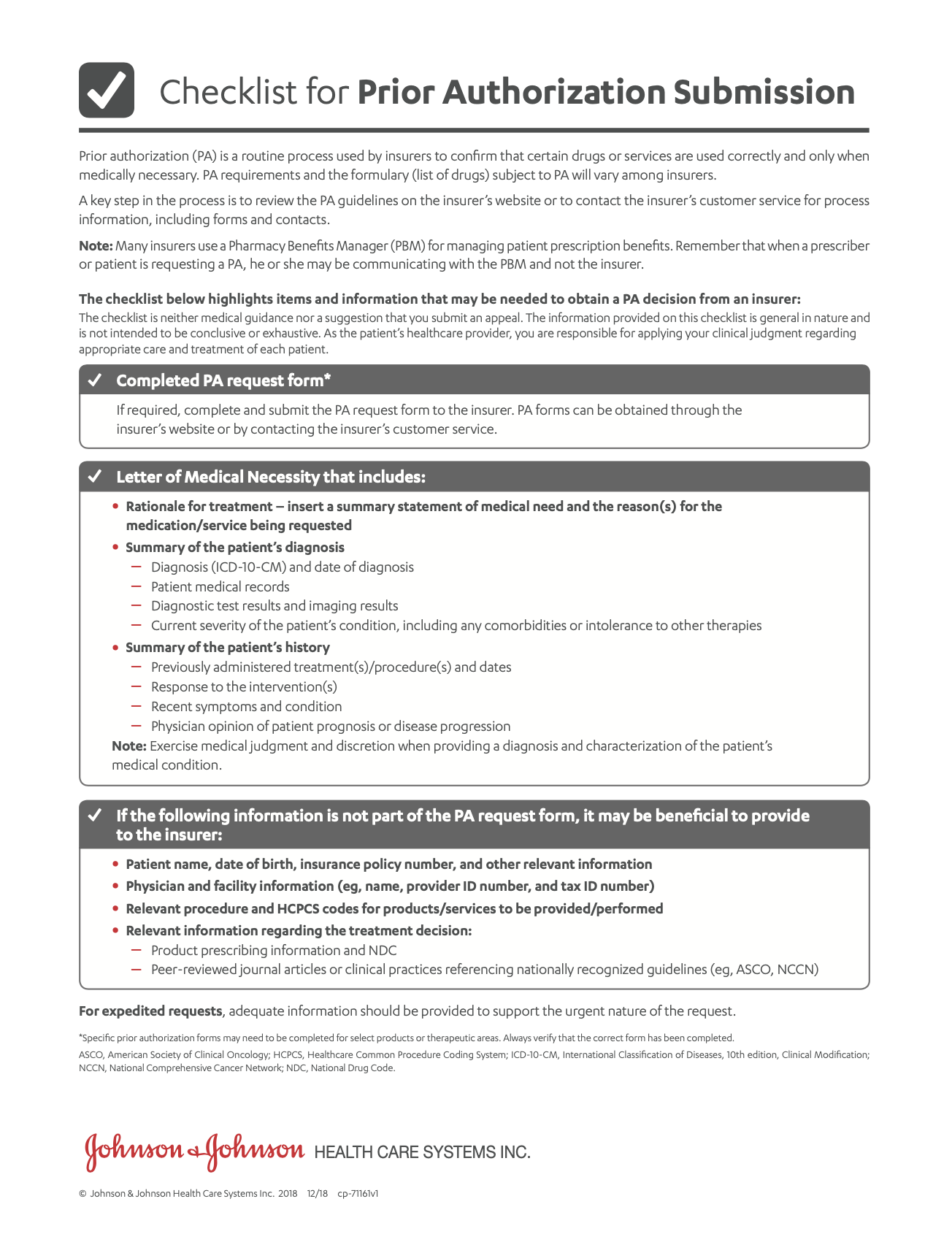
Contraindications
- Darunavir and cobicistat are both inhibitors and substrates of the cytochrome P450 3A (CYP3A) isoform. PREZCOBIX® should not be co-administered with medicinal products that are highly dependent on CYP3A for clearance and for which increased plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). In addition, co-administration of PREZCOBIX® with CYP3A inducers may lead to lower exposures of darunavir and cobicistat, and potential loss of efficacy of darunavir and possible resistance.
Examples of drugs that are contraindicated for co-administration with PREZCOBIX® are: alfuzosin, carbamazepine, colchicine (in patients with renal and/or hepatic impairment), dihydroergotamine, dronedarone, elbasvir/grazoprevir, ergotamine, ivabradine, lomitapide, lovastatin, lurasidone, methylergonovine, oral midazolam, naloxegol, phenobarbital, phenytoin, pimozide, ranolazine, rifampin, St. John’s wort (Hypericum perforatum), sildenafil for pulmonary arterial hypertension, simvastatin, and triazolam.
Warnings and Precautions
-
Hepatotoxicity: Patients with pre-existing liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities, including severe hepatic adverse reactions. Drug-induced hepatitis and cases of liver injury, including some fatalities, have been reported.
Appropriate laboratory testing should be conducted prior to initiating and during therapy with PREZCOBIX®. Evidence of new or worsening liver dysfunction in patients on PREZCOBIX® should prompt consideration of interruption or discontinuation of treatment.
-
Severe Skin Reactions: Severe skin reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis have been reported in patients receiving darunavir coadministered with ritonavir. Mild-to-moderate rash was also reported and often occurred and resolved with continued dosing. Discontinue PREZCOBIX® immediately if signs or symptoms of severe skin reaction develop.
-
Sulfa Allergy: Monitor patients with a known sulfonamide allergy after initiating PREZCOBIX®.
-
Effects on Serum Creatinine: Cobicistat decreases estimated creatinine clearance (CrCl) due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function. Prior to starting PREZCOBIX®, assess estimated CrCl. Patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg/dL from baseline should be closely monitored for renal safety. Consider alternative medications that do not require dosage adjustments in patients with renal impairment.
-
Renal Impairment When Used With Tenofovir Disoproxil Fumarate (DF): Renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported with the use of tenofovir DF and cobicistat. Coadministration with tenofovir DF is not recommended in patients who have an estimated CrCl <70 mL/min. In all patients, monitor estimated CrCl, urine glucose, and urine protein prior to initiating and during therapy. Measure serum phosphorus in patients at risk of renal impairment. Coadministration of PREZCOBIX® and tenofovir DF in combination with concomitant or recent use of a nephrotoxic agent is not recommended.
-
Risk of Serious Adverse Reactions or Loss of Virologic Response Due to Drug Interactions: Initiation of PREZCOBIX®, which inhibits CYP3A, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving PREZCOBIX® may increase plasma concentrations of medications metabolized by CYP3A and reduce plasma concentrations of active metabolite(s) formed by CYP3A. Initiation of medications that inhibit or induce CYP3A may respectively increase or decrease concentrations of PREZCOBIX®. These interactions may lead to clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from higher exposures of concomitant medications; clinically significant adverse reactions from higher exposures of PREZCOBIX®; loss of therapeutic effect of concomitant medications from lower exposures of active metabolite(s); or loss of therapeutic effect of PREZCOBIX® and possible development of resistance from lower exposures of PREZCOBIX®.
-
Antiretrovirals Not Recommended: Do not use PREZCOBIX® in combination with other antiretroviral drugs that require pharmacokinetic boosting or which contain the individual components of PREZCOBIX® (darunavir and cobicistat) or with ritonavir.
-
Diabetes Mellitus/Hyperglycemia and Hemophilia: New onset or exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported in patients receiving protease inhibitors. Initiation or dose adjustments of insulin or oral hypoglycemic agents may be required. Increased bleeding in hemophiliacs has been reported in patients receiving protease inhibitors.
-
Fat Redistribution: Redistribution and/or accumulation of body fat have been observed in patients receiving antiretroviral therapy.
-
Immune Reconstitution Syndrome including the occurrence of autoimmune disorders with variable time to onset has been reported.
Adverse Reactions
- The most common clinical adverse reactions (incidence ≥5%) of at least moderate intensity (≥Grade 2) were diarrhea, nausea, rash, headache, abdominal pain, and vomiting during the darunavir clinical development program, where darunavir was coadministered with ritonavir.
This is not a complete list of all adverse drug reactions reported with the use of PREZCOBIX®. Please refer to the full Prescribing Information for a complete list of adverse drug reactions.
Drug Interactions
- Consult the full Prescribing Information for PREZCOBIX® for information on potentially significant drug interactions, including clinical comments.
Use in Specific Populations
- PREZCOBIX® is not recommended for use during pregnancy because of substantially lower exposures of darunavir and cobicistat during pregnancy.
- PREZCOBIX® should not be initiated in pregnant individuals. An alternative regimen is recommended for those who become pregnant during therapy with PREZCOBIX®.
Lactation: The Centers for Disease Control and Prevention recommends that HIV-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection.
- The safety and effectiveness of PREZCOBIX® have not been established and is not recommended in pediatric patients weighing less than 40 kg.
- Consult the full Prescribing Information for PREZCOBIX® for additional information on the Uses in Specific Populations.
Please see full Prescribing Information for more details.
cp-08641v12