ICD-10 Codes
- Access & Reimbursement GuideA comprehensive summary of important medication information including uses, Important Safety Information, access, and reimbursement.
- Benefits Investigation FormA way to find out if RYBREVANT® is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies.
- Business Associate AgreementComplete a Business Associate Agreement for your practice only once. No individual patient authorizations are required.
- Coding & Billing Guide
- Exception Considerations ChecklistA guide to submitting a formulary exception request.
Exception Considerations Checklist (en español) - FDA Approval Letter for RYBREVANT®
- Janssen CarePath Resource GuideA comprehensive summary of support tools for your office to help patients start and stay on treatment.
- Letter of ExceptionA template that you can fill out and submit to a patient’s health insurance provider asking them to cover a medication that is not on formulary.
- Letter of Medical NecessityA template that you can fill out and submit to a patient’s health insurance provider. You may use it to explain why RYBREVANT® is medically necessary for your patient.
- Patient Account Overview
- Patient Affordability OptionsDiscover options that can make RYBREVANT® more affordable for your patients.
- Patient Authorization FormIndividual patient form for offices without a Business Associate Agreement.
Patient Authorization Form (en español) - Patient Authorization Form (en español)Individual patient form for offices without a Business Associate Agreement.
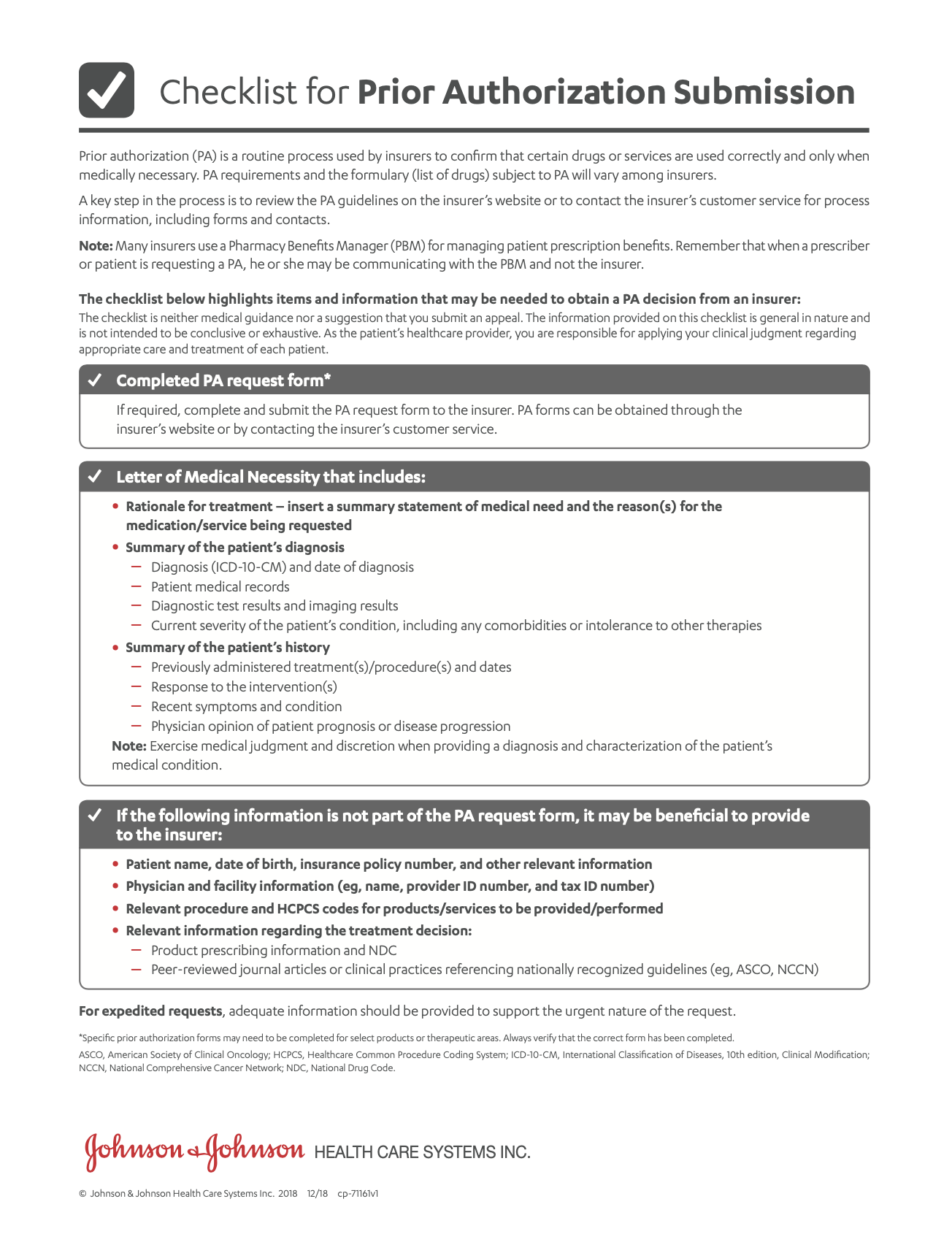
- Prior Authorization Considerations ChecklistA checklist to guide you through the prior authorization process.
Prior Authorization Considerations Checklist (en español) - Savings Program (Overview)Eligible patients using commercial or private insurance can save on out-of-pocket costs for RYBREVANT®.
- Savings Program Assignment of Benefits FormA form the patient can submit that allows Janssen CarePath Savings Program to reimburse the provider directly.
- Savings Program – Submitting Medical ClaimsA guide on submitting medical benefit rebate claims for RYBREVANT®.
- Verification of Benefits Guide (Medical)A guide to understanding the Verification of Benefits for your patient’s medical benefits.
- Verification of Benefits Guide (Pharmacy)A guide to understanding the Verification of Benefits for your patient’s pharmacy benefits.

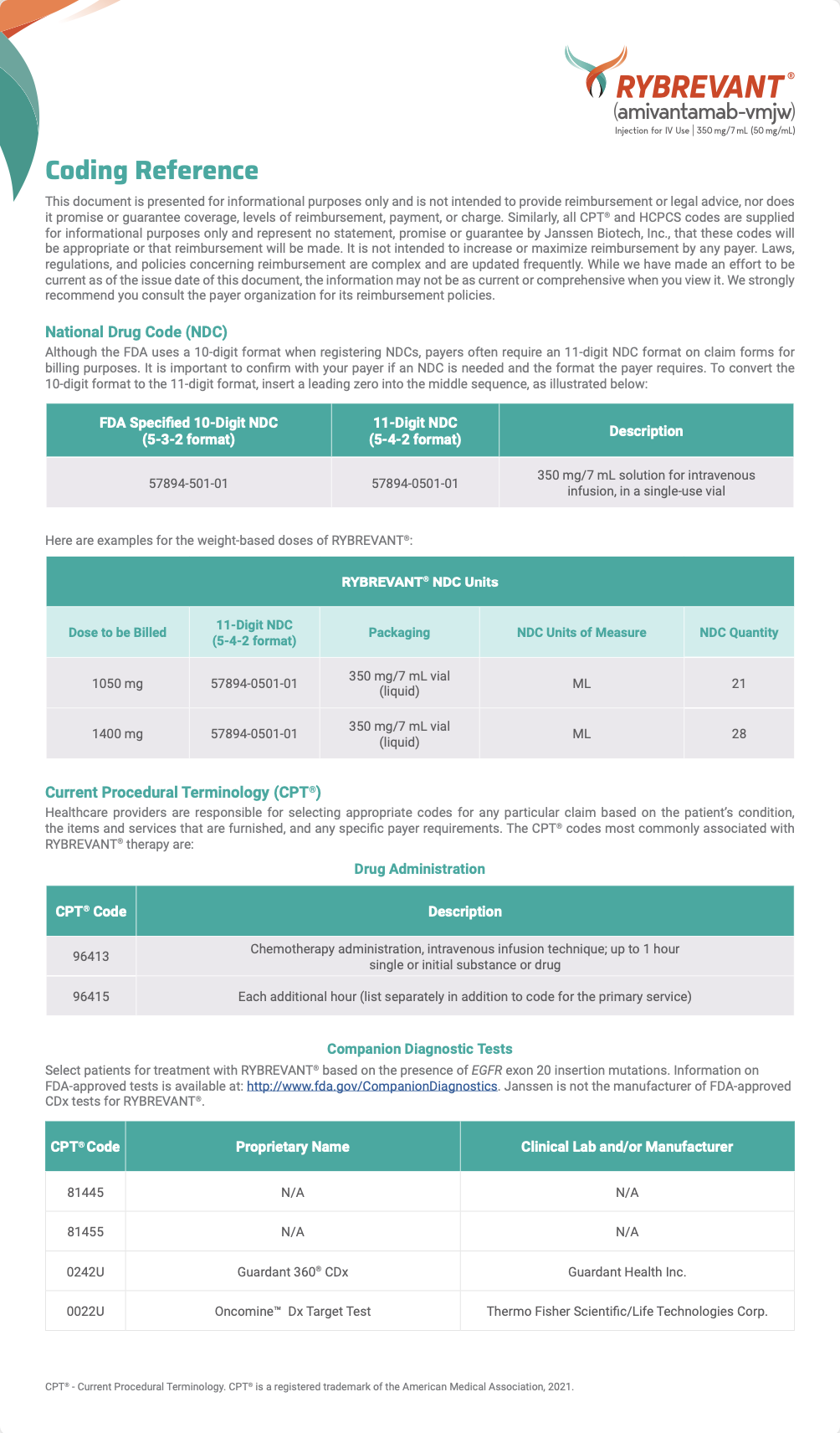
ICD-10 Codes
C34 Malignant Neoplasm of Bronchus or Lung
| ICD-10 Indication | ICD-10 Code |
| Malignant neoplasm of bronchus and lung | C34 |
| Malignant neoplasm of upper lobe, bronchus or lung | C34.1 |
| Malignant neoplasm of upper lobe, unspecified bronchus or lung | C34.10 |
| Malignant neoplasm of upper lobe, right bronchus or lung | C34.11 |
| Malignant neoplasm of upper lobe, left bronchus or lung | C34.12 |
| Malignant neoplasm of middle lobe, bronchus or lung | C34.2 |
| Malignant neoplasm of lower lobe, bronchus or lung | C34.3 |
| Malignant neoplasm of lower lobe, unspecified bronchus or lung | C34.30 |
| Malignant neoplasm of lower lobe, right bronchus or lung | C34.31 |
| Malignant neoplasm of lower lobe, left bronchus or lung | C34.32 |
| Malignant neoplasm of overlapping sites of bronchus and lung | C34.8 |
| Malignant neoplasm of overlapping sites of unspecified bronchus and lung | C34.80 |
| Malignant neoplasm of overlapping sites of right bronchus and lung | C34.81 |
| Malignant neoplasm of overlapping sites of left bronchus and lung | C34.82 |
| Malignant neoplasm of unspecified part of bronchus or lung | C34.9 |
| Malignant neoplasm of unspecified part of unspecified bronchus or lung | C34.90 |
| Malignant neoplasm of unspecified part of right bronchus or lung | C34.91 |
| Malignant neoplasm of unspecified part of left bronchus or lung | C34.92 |
Collected in 10/21 and may change.
This information is not a promise of coverage or payment. It is not intended to give reimbursement advice or increase reimbursement by any payer. Legal requirements and plan information can be updated frequently. Contact the plan for more information about current coverage, reimbursement policies, restrictions, or requirements that may apply.
For more information on ICD-10, visit the CMS website.
SOURCE
ICD-10-CM 2022: The Complete Official Codebook. American Medical Association, 2021.