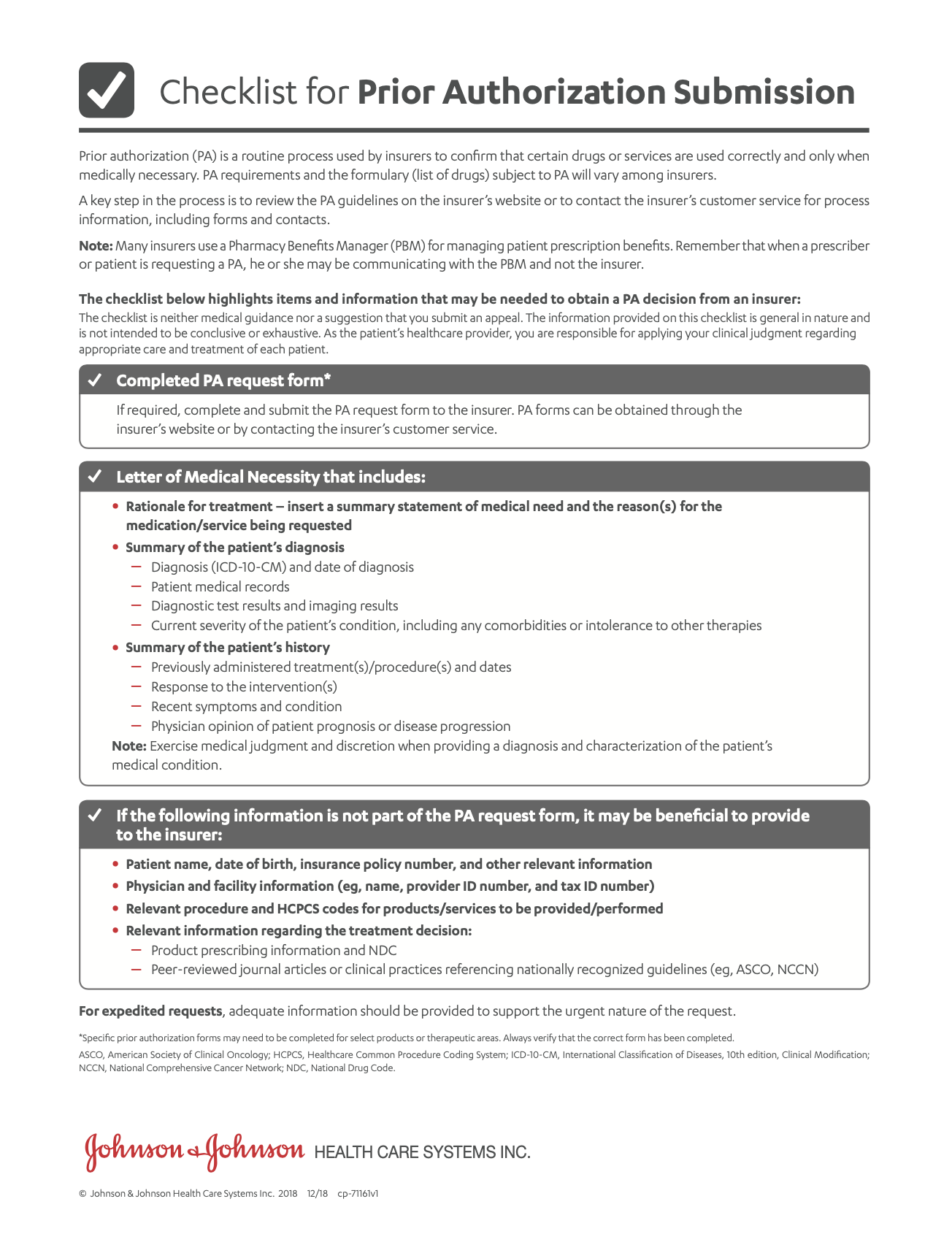
Help Your Patients Start and Stay on TALVEY™
- Access and Reimbursement GuideA comprehensive summary of important medication information including uses, Important Safety Information, access, and reimbursement.
- Benefits Investigation FormA way to find out if TALVEY™ is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies.
- Business Associate AgreementComplete a Business Associate Agreement for your practice only once. No individual patient authorizations are required.
- Coding and Billing Guide
- Exception Considerations ChecklistA guide to submitting a formulary exception request.
Exception Considerations Checklist (en español) - Exception Considerations Checklist (en español)A guide to submitting a formulary exception request.
- FDA Approval Letter for TALVEY™
- Janssen CarePath Resource GuideA comprehensive summary of support tools for your office to help patients start and stay on treatment.
- Letter of Medical Necessity and ExceptionA template that you can fill out and submit to a patient’s health insurance provider. You may use it to explain why TALVEY™ is medically necessary for your patient or to request a formulary exception.
- Patient Account Overview
- Patient Affordability OptionsDiscover options that can make TALVEY™ more affordable for your patients.
- Patient Authorization FormIndividual patient form for offices without a Business Associate Agreement.
Patient Authorization Form (en español) - Patient Authorization Form (en español)Individual patient form for offices without a Business Associate Agreement.
- Prior Authorization Considerations ChecklistA checklist to guide you through the prior authorization process.
Prior Authorization Considerations Checklist (en español) - Prior Authorization Considerations Checklist (en español)A checklist to guide you through the prior authorization process.
- Savings Program (Overview)Eligible patients using commercial or private insurance can save on out-of-pocket costs for TALVEY™.
- Savings Program Assignment of Benefits FormA form the patient can submit that allows Janssen CarePath to reimburse the provider directly.
- Savings Program Rebate FormA form the patient can submit if the pharmacy is not able to process the Janssen CarePath Savings Program card.
- Savings Program – Submitting Medical ClaimsA guide on submitting medical benefit rebate claims for TALVEY™.
- Specialty Distributors for TALVEY™ Flashcard
- Verification of Benefits Guide (Medical)A guide to understanding the Verification of Benefits for your patient’s medical benefits.
- Verification of Benefits Guide (Pharmacy)A guide to understanding the Verification of Benefits for your patient’s pharmacy benefits.

Help Your Patients Start and Stay on TALVEY™
Janssen Compass®
Personalized 1-on-1 Support for Your Patients
Starting and staying on track with a new medication can feel overwhelming for patients. Janssen Compass® Care Navigators are here to help by offering free, personalized 1-on-1 support throughout their treatment journey.
Each patient who enrolls in the program will be paired with a dedicated Care Navigator. Their Care Navigator will partner with them to schedule check-ins via the phone.
On every phone call, patients will speak to their dedicated Care Navigator who will help guide them in 3 key areas:
-
Cost and Affordability: Discover resources that may be able to help patients afford their Janssen medication – no matter what type of insurance they have:
- Commercially insured: Enroll eligible patients in Janssen CarePath Savings Program
- Government-funded coverage: Determine Medicare Part D low-income subsidy (LIS) eligibility; refer to government resources or independent foundations
- Uninsured and underinsured: Connection to independent foundations and other resources.
-
Medication and Disease Education: Educate patients to help them start and stay on the prescribed treatment plan, including:
- Understanding their disease
- Reinforcing dosing and administration for their Janssen medication
- Conversation guides to facilitate communication with their care team
-
Practical and Emotional Support: Empower patients and caregivers with lifestyle and coping skills to help them manage stress. Plus, connect them to advocacy organizations for their practical and emotional support needs, which may include:
- Finding support groups
- Connection to transportation-related services
- Health and wellness strategies
Get your patient connected with a Care Navigator today
-
Call the program at 844-628-1234, Monday through Friday, 8:30 AM–8:30 PM ET
OR - Schedule your patient for an introductory call from a Care Navigator here: JanssenCompass.com/signup
Janssen Compass® is limited to education for patients about their Janssen therapy, its administration, and/or their disease. It is intended to supplement a patient’s understanding of their therapy and is not intended to provide medical advice, replace a treatment plan from the patient’s doctor or nurse, provide case management services, or serve as a reason to prescribe a Janssen medication.
Janssen CarePath Patient Account
Your patients and their caregivers can create an online account at MyJanssenCarePath.com where they can learn about their insurance coverage.
They can also:
- Enroll in the Janssen CarePath Savings Program
- Manage their benefits
- Sign up for treatment reminders
- Find support throughout their treatment journey
Call a Janssen CarePath Care Coordinator at 877-CarePath (877-227-3728), Monday−Friday, 8:00 AM to 8:00 PM ET. Multilingual phone support available.
Sign up or log in to the Provider Portal at JanssenCarePathPortal.com where you can request and review benefits investigations, enroll eligible patients in the Janssen CarePath Savings Program, and view their Savings Program transactions.